Chapter 1
Mater In Our Surroundings
Page-3
Questions-
- Which of the following are matter?
Chair, air, love, smell, hate, almonds, thought, cold, lemon water, smell of perfume.
Ans-
- Give reasons for the following observation:
The smell of hot sizzling food reaches you several metres away, but to get the smell from cold food you have to go close.
Ans-
- A diver is able to cut through water in a swimming pool. Which property of matter does this observation show?
Ans-
- What are the characteristics of the particles of matter?
Ans-
Page-6
Questions-
- The mass per unit volume of a substance is called density.
(denisty = mass / volume). Arrange the following in order of increasing density – air, exhaust from chimneys, honey water, chalk cotton and iron.
Ans-
- (a) Tabulate the differences in the characteristics of states of matter.
(b) Comment upon the following: rigidity, compressibility, fluidity, filling a gas container, shape, kinetic energy and denisty.
Ans-
- Give reasons
(a) A gas fills completely the vessel in which it is kept.
(b) A gas exerts pressure on the walls of the container.
(c) A wooden table should be called a solid.
(d) We can easily move our hand in air but to do the same through a solid black of wood we need a karate expert.
Ans-
- Liquids generally have lower density as compared to solids. But you must have observed that ice floats on water. Find out why.
Ans-
Page-9
Questions-
- convert the following temperature to celsius scale.
a. 300k b. 573k.
Ans-
- What is the physical state of water at:
a. 250ºC b. 100ºC
Ans-
- For any substance. why does the temperature remain constant during the change of state?
Ans-
- Suggest a method to liquefy atmospheric gasses.
Ans-
Page-10
Questions-
- Why does a desert cooler cool better on a hot dry day?
Ans-
- How does the water kept in an earthen pot (matka) become cool during summer?
Ans-
- Why does our palm feel cold when we put some acetone or petrol or perfume on it?
Ans-
- Why are we able to sip hot tea or milk faster from a saucer rather than a cup?
Ans-
- What type of clothes should we wear in summer?
Ans-
Page-12
Exercises-
- Convert the following temperatures to the celsius scale.
(a) 293 K (b) 470 K
- Convert the following temperatures to the kelvin scale.
(a) 25° C (b) 373° C
- Give reason for the following observations.
(a) Naphthalene balls disappear with time without leaving any solid.
(b) We can get the smell of perfume sitting several metres away.
Ans-
- Arrange the following substances in increasing order of forces of attraction between the particles- Water, Sugar, Oxygen.
Ans-
- What is the physical state of water at-
(a) 25ºC (b) 0ºC (c) 100ºC
(ক) 25ºC (খ) 0ºC (গ) 100ºC
Ans-
- Give two reasons to justify-
(a) Water at room temperature is liquid.
(b) an iron almirah is a solid at room temperature.
Ans-
- Why is ice at 273 k more effective in cooling than water or steam?
Ans-
- What produces more severe burns, boiling water or steam?
Ans-
- Name A, B, C, D, E And F in the following diagram showing change in its state
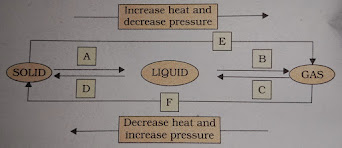
Ans: